1. What is Valence Bond (VB) Theory?
This lecture provides a brief introduction to Valence Bond theory, aiming to give you a basic impression of its concepts. Some terms may seem unfamiliar at first—there is no need to worry, as they will be explained in detail in later chapters.
1.1. Introduction to Classical Valence Bond Theory
First, let us think about and try to answer the following question:
Consider
How does the H2 molecule form a chemical bond?
For this question, the explanations provided by Valence Bond (VB) theory and Molecular Orbital (MO) theory differ in some respects. According to the mainstream Molecular Orbital theory, the explanation is as follows:
The \(1s\)atomic orbitals of the two H atoms, due to their similar energies and symmetry matching, can linearly combine to form two molecular orbitals: a bonding orbital and an antibonding orbital.
These two molecular orbitals are delocalized and orthogonal to each other. The energy of the bonding orbital is lower than that of the atomic orbitals, while the energy of the antibonding orbital is higher.
The two electrons of the H2 molecule occupy the molecular orbitals in order of increasing energy; therefore, both electrons fill the bonding orbital, and the antibonding orbital remains unoccupied. As a result, a chemical bond is formed between the two H atoms, and the total energy of the system is lower than that of the separated H atoms.
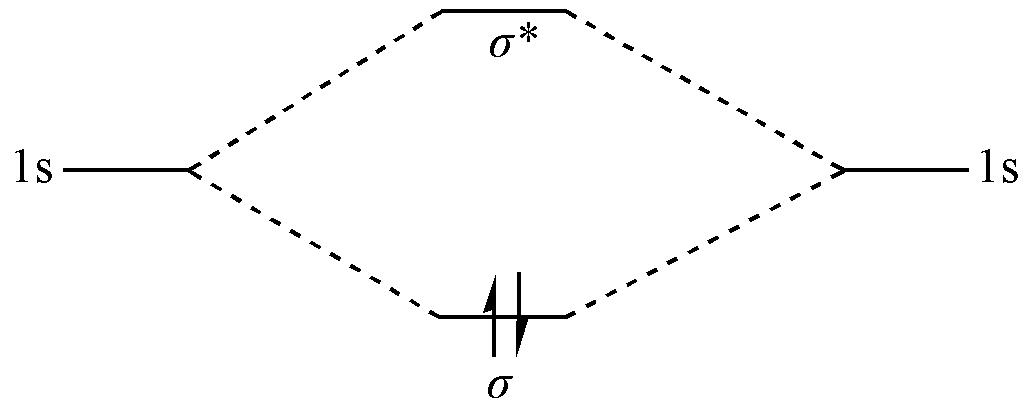
Fig. 1.1.1 Bonding mechanism of H2 in Molecular Orbital theory
As can be seen, in Molecular Orbital theory, electrons are inherently delocalized in the system. Localization is only a special case of delocalization. The formation of a chemical bond arises because there are more electrons in the bonding orbital than in the antibonding orbital.
Hint
When we say that an orbital is localized or delocalized, we refer to the spatial extent of the electron cloud when electrons occupy this orbital. A localized orbital means its corresponding electron cloud is confined to a certain region; a delocalized orbital means its electron cloud can spread over the entire system.
In Valence Bond (VB) theory, the bonding mechanism of the H2 molecule is described as follows:
The two hydrogen atomic orbitals are localized and non-orthogonal, each being localized on its respective H atom. The electrons in the system pair with antiparallel spins:
The two electrons in H₂ are shared between the two hydrogen atoms. Consequently, there are multiple possible configurations for the electrons to occupy the localized atomic orbitals. The electrons may occupy each atomic orbital separately, or they may both occupy the same atomic orbital. These different occupancy patterns constitute distinct valence bond structures.
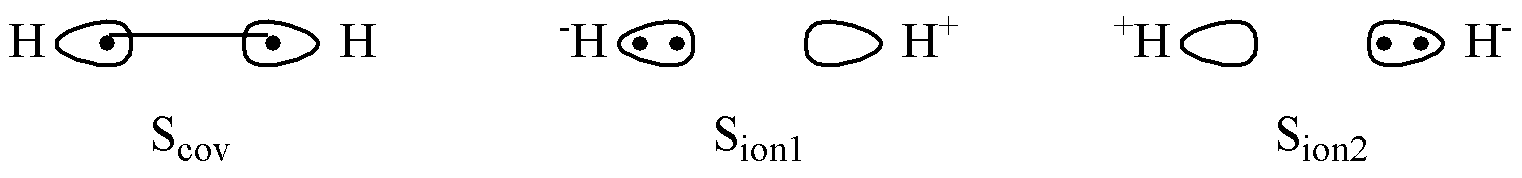
According to quantum mechanics, these valence bond structures each contribute with a certain probability to the final state of the H2 molecule, forming a resonance. Furthermore, when the two electrons occupy separate orbitals with antiparallel spins, there exists an exchange interaction between them.
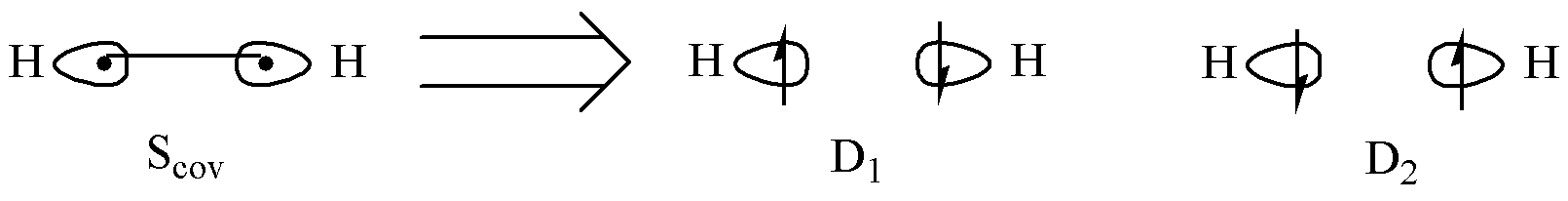
Attention
Because valence bond structures are closely related to resonance, they are sometimes also called resonance structures. In the following discussion, we will not deliberately distinguish between the two terms.
As can be seen, from the perspective of VB theory, chemical bonds are localized, whereas delocalization is only a special case of localization. Chemical bonding arises through electron exchange and resonance, enabled by the pairing of two antiparallel-spin electrons between the bonding atoms.
These differences can be summarized in the following table:
VB |
MO |
|
|---|---|---|
Whether the bonding is localized |
Yes |
No |
Whether the orbitals are orthogonal |
No |
Yes |
Origin of bonding |
Electron exchange and structural resonance |
More electrons in bonding orbital than in antibonding orbital |
At this point, beginners may already feel confused by concepts such as structure and resonance. That is perfectly fine—just keep them in mind for now, and we will explain their precise meaning in later chapters.
To summarize, classical valence bond theory is a bonding theory that uses localized, non-orthogonal atomic orbitals, and describes chemical bonds in terms of resonance structures formed by different electron occupancy patterns. It provides a framework to explain chemical bonding and elucidate reaction mechanisms.
1.2. Characteristics of Valence Bond (VB) Theory: Advantages and Limitations
How atoms form chemical bonds has always been a central concern for chemists. Since the publication of Lewis’s famous paper, the concept of electron pairing in bond formation has gradually become widely accepted. The studies by Heitler and London on H2 bonding, as well as Pauling’s introduction of resonance theory, marked the final maturation of Valence Bond theory. The greatest advantage of VB theory lies in its intuitive nature. The concept of localized bonding in VB theory aligns with the intuitive understanding of chemical bonds, and valence bond structures provide a concrete representation of this bonding idea. Through these structures, one can conveniently investigate and understand changes in the electronic structure during chemical reactions—such as atomic orbitals, electron occupancy, and bond polarity. This level of intuitive insight is difficult to achieve with Molecular Orbital theory. Therefore, in the early days (before the 1950s), VB theory was the primary tool for discussing reaction mechanisms and explaining chemical phenomena.
However, with the development of computational chemistry, MO theory gradually replaced VB theory as the primary tool. The main reason is the computational complexity of VB calculations. Even for a simple H2 molecule, VB theory requires multiple valence bond structures (which will be discussed in detail for given systems in later chapters) to describe the bond. Moreover, the use of localized, non-orthogonal orbitals in VB theory further increases computational cost. As the number of bonds in a chemical system increases, the computational effort grows dramatically. In the early days, such large computational requirements often made quantitative calculations infeasible. Meanwhile, MO theory maintained a simpler formalism and manageable computational cost. As a result, VB theory gradually became primarily a qualitative tool. During this period, several improved VB methods were developed, such as Generalized Valence Bond (GVB) and Spin-Coupled Valence Bond (SCVB). Unlike traditional VB methods, these approaches employ delocalized orbitals and some orthogonalization to reduce computational effort. However, the delocalization of orbitals also reduces the precision of valence bond structures in describing chemical bonds. We usually refer to VB methods using delocalized orbitals as modern VB theory, and methods using localized orbitals as classical VB theory.
Fortunately, with advances in computer technology, the computational cost of classical VB theory has become more manageable. Since the 1980s, with the development of Valence Bond Self-Consistent Field (VBSCF) methods, ab initio calculations based on classical VB theory became feasible. Subsequent development of methods such as Breathing Orbital Valence Bond (BOVB), Valence Bond Configuration Interaction (VBCI), and Valence Bond Second-Order Perturbation (VBPT2) made high-accuracy calculations using classical VB theory a reality.
1.3. Common Misconceptions about Valence Bond Theory
Another important reason why MO theory replaced VB theory as the mainstream is misunderstandings of VB theory, particularly resonance theory. A classic example is the explanation of the ground state of O2. Traditional resonance theory suggests that the bonding in O2 should be represented by the following pattern:
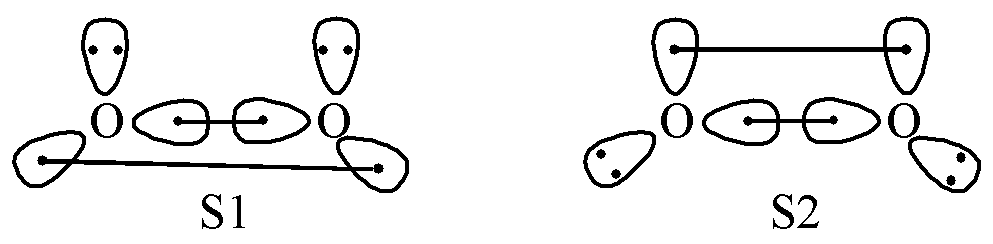
Fig. 1.3.1 Bonding mechanism of O2 in traditional resonance theory
In this representation, O2 has two bonds: one \(\sigma\)bond and one \(\pi\) bond. According to this bonding model, O2 should have no unpaired electrons and be diamagnetic. However, experiments show that although the O2 molecule has a double bond, its ground state is paramagnetic, indicating the presence of unpaired electrons. Meanwhile, MO theory provides a different bonding picture (ignoring \(1s\)and \(2s\)orbitals that do not affect the bonding analysis):
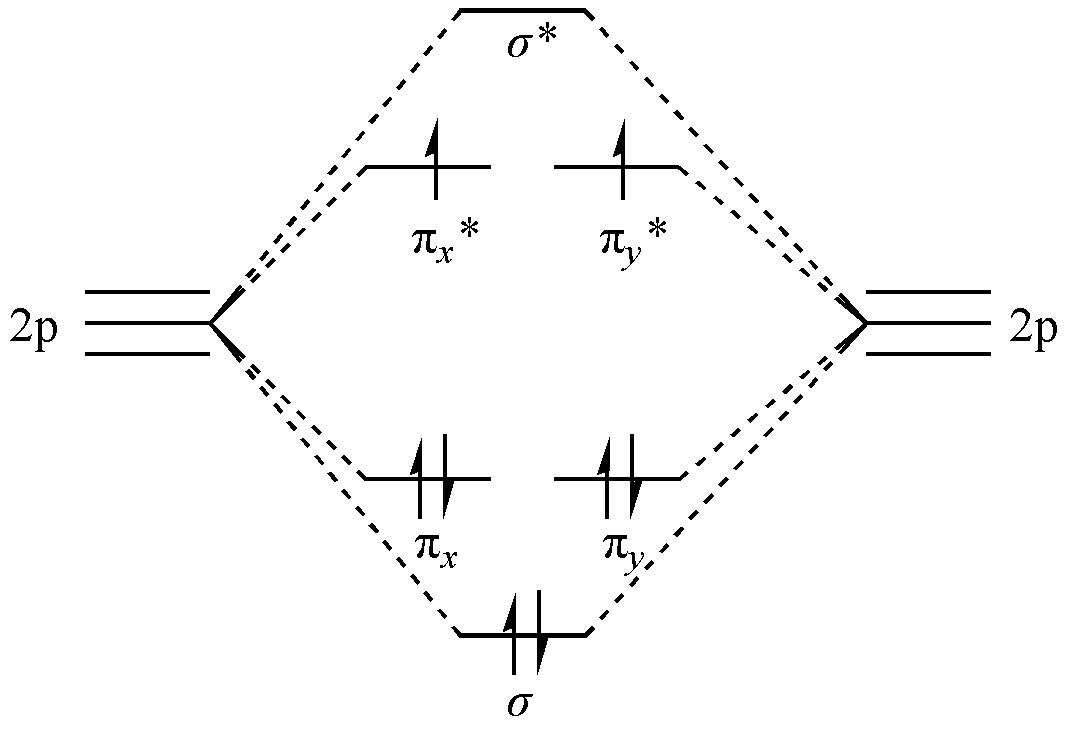
Fig. 1.3.2 Bonding mechanism of O₂ in Molecular Orbital theory
As shown in Fig. 1.3.2, six p orbitals combine linearly to form molecular orbitals. The eight p electrons occupy these orbitals according to the Aufbau principle and Hund’s rule, resulting in a triplet ground state with a total bond order of 2, consistent with a double bond between the O atoms.
MO theory perfectly explains the bonding in O2, which caused some chemists to question the validity of VB theory. The problem arose because early descriptions attempted to represent O2 bonding using oversimplified resonance structures. In fact, Pauling had already proposed that the bonding in O2 along the \(\sigma\)direction should not be simple single bonds, but rather two 3-electron-2-center bonds, as illustrated below:
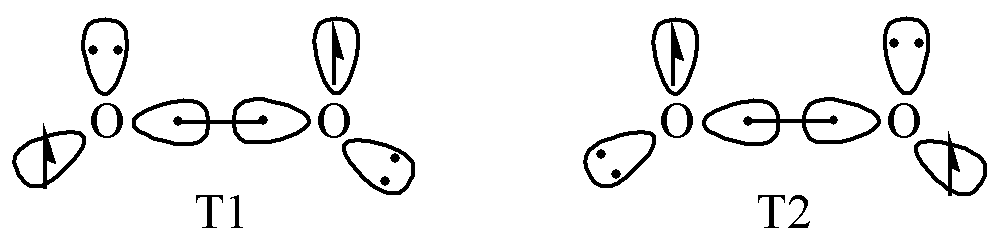
Fig. 1.3.3 Bonding mechanism of O2 in Valence Bond theory
In 2007, Su Peifeng and co-workers at Xiamen University demonstrated this through valence bond calculations on the O2 molecule. This shows that valence bond theory remains both valid and accurate.
1.4. When Should Valence Bond Theory Be Used?
Although this course focuses on VB theory, it is important to emphasize that not all calculations require VB theory. Today, the representations provided by MO theory are widely accepted, supported by a variety of computational methods, extensive software, and high computational efficiency. MO theory provides reliable results for thermodynamic properties, reaction processes, excited states, and spectroscopic properties. Therefore, there is generally no need to adopt VB theory for novelty’s sake. VB theory becomes relevant only when your research is closely related to chemical bonding, and MO theory fails to provide satisfactory explanations.